
Not just a study that includes AI, but any clinical study needs a crystal-clear protocol that defines how a study will be conducted in its finest detail.
It is important to use guidelines such as SPIRIT that give the writer a list of checkboxes to be included.
As such, the SPIRIT committee has published an Extension specific to AI clinical studies that include the original guidelines. In total, 15 items (12 extensions and 3 elaborations) were addressed for trial protocols of AI interventions.
Here is the list of items that need to be covered. If you would like to download an editable .doc version of the list, please click here.
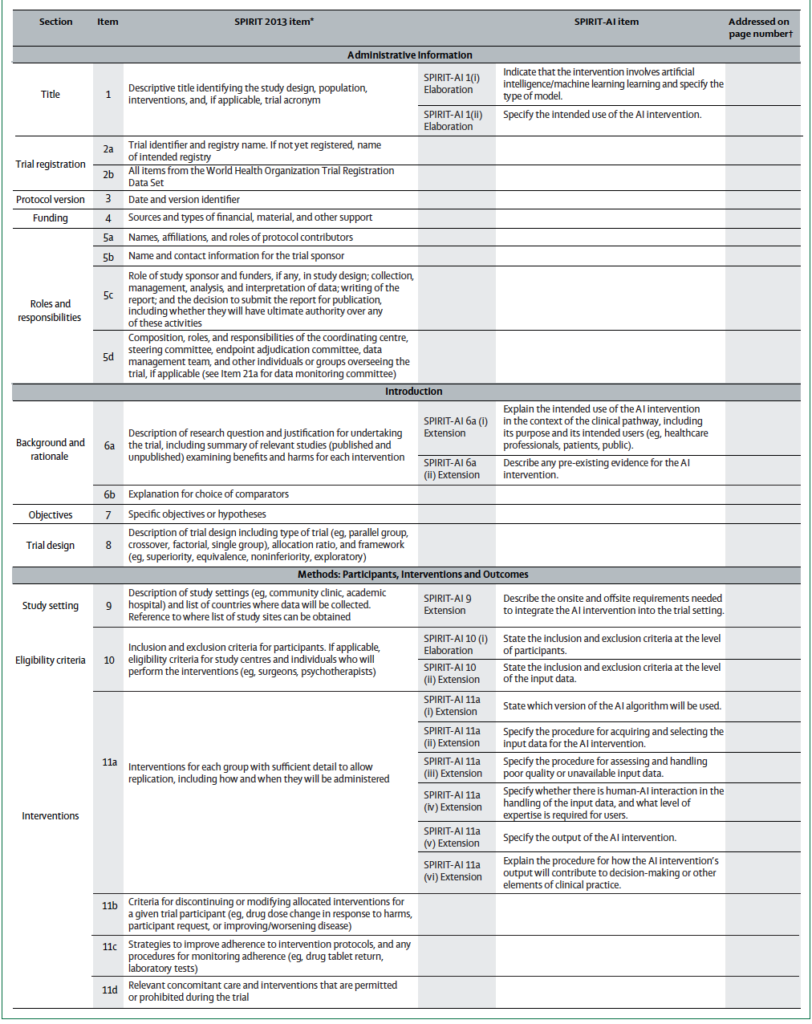
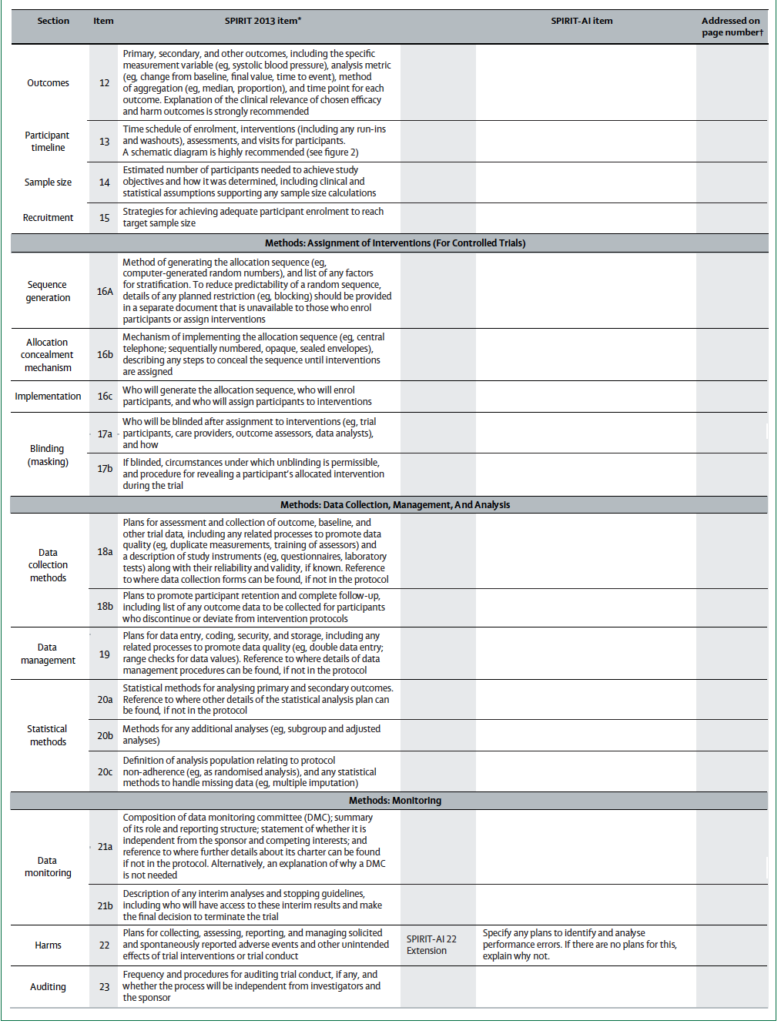

The 15 items are listed below.
Administrative information
SPIRIT-AI 1 (i) Elaboration: Indicate that the intervention involves artificial intelligence/machine learning and specify the type of model
SPIRIT-AI 1 (ii) Elaboration: Specify the intended use of the AI intervention
Introduction
SPIRIT-AI 6a (i) Extension: Explain the intended use of the AI intervention in the context of the clinical pathway, including its purpose and its intended users (for example, health-care professionals, patients, public)
SPIRIT-AI 6a (ii) Extension: Describe any pre-existing evidence for the AI intervention
Participants, interventions, and outcomes
SPIRIT-AI 9 Extension: Describe the onsite and offsite requirements needed to integrate the AI intervention into the trial setting
SPIRIT-AI 10 (i) Elaboration: State the inclusion and exclusion criteria at the level of participants
SPIRIT-AI 10 (ii) Extension: State the inclusion and exclusion criteria at the level of the input data
SPIRIT-AI 11a (i) Extension: State which version of the AI algorithm will be used
SPIRIT-AI 11a (ii) Extension: Specify the procedure for acquiring and selecting the input data for the AI intervention
SPIRIT-AI 11a (iii) Extension: Specify the procedure for assessing and handling poor-quality or unavailable input data
SPIRIT-AI 11a (iv) Extension: Specify whether there is human– AI interaction in the handling of the input data, and what level of expertise is required for users
SPIRIT-AI 11a (v) Extension: Specify the output of the AI intervention
SPIRIT-AI 11a (vi) Extension: Explain the procedure for how the AI intervention’s outputs will contribute to decision-making or other elements of clinical practice
Monitoring
SPIRIT-AI 22 Extension: Specify any plans to identify and analyse performance errors. If there are no plans for this, explain why not
Ethics and dissemination
SPIRIT-AI 29 Extension: State whether and how the AI intervention and/or its code can be accessed, including any restrictions to access or re-use
Important:
These items should be routinely reported in addition to the core SPIRIT 2013 checklist items.
Flow Diagram adopted for AI study protocol (BEFORE and AFTER) image.
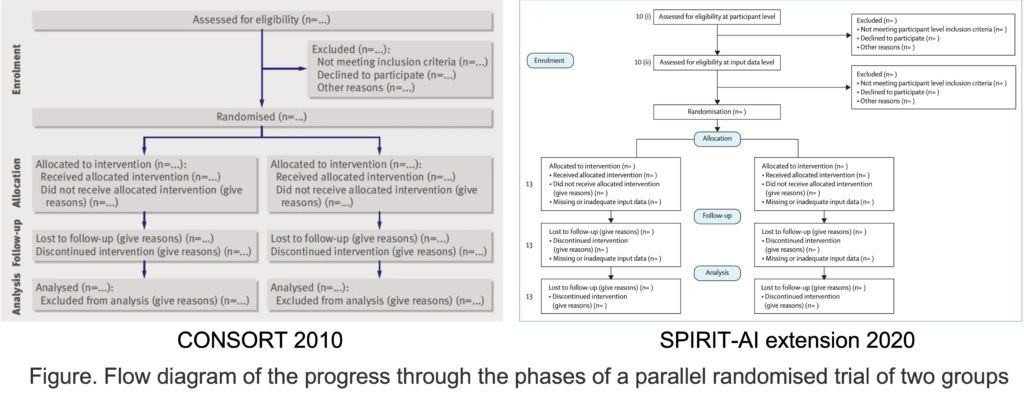
For fillable form, please click here.
References:
- Lancet Digital Health 2020; e549–560
- BMJ 2010;340:c869
- Ann Intern Med. 2013 Feb 5;158(3):200-7.
I was more than happy to discover this site. I wanted to thank you for your time just for this fantastic read!! I definitely really liked every little bit of it and I have you book-marked to look at new information in your website.
Pingback: Can AI fix the 50Billion$ clinical trial market? - Digital health pulse